Our Medical Electronics Expertise
Over 30 years’ experience with medical devices
Specialized design in Electronic Medical Devices
MAATEL has NF EN ISO 13485 (V.2012) certification and a wealth of expertise in product compliance for European certification and accreditation by the FDA (Foods and Drugs Administration), the public body responsible for the safety of drugs and medical devices in the United States.
MAATEL’s experience is built on the design and production of electronic medical devices for over 30 years.
| MD AIMD IVDD | Dir 93/42/EEC (class l, lla, llb, lll) Dir 90/385/EEC Dir 98/79/EC (class A, B, C, D) |

Our creations
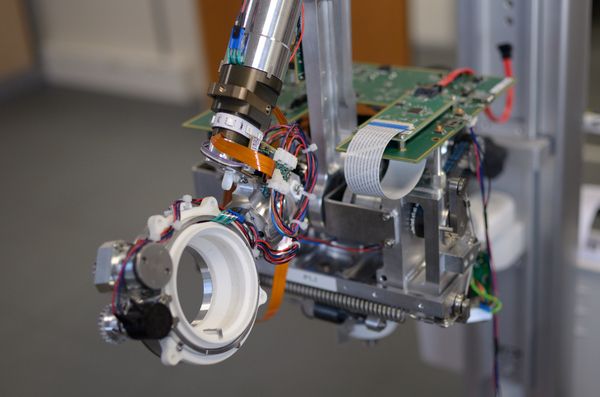
- Class I medical device
- Class C software
- Design of digital and analog circuit boards, motor controls, 6 axes Embedded Linux, real-time Cortex M7
- High-speed USB and Ethernet connection
- Direct current (DC) driven motors, with torque
- Redundancy sensors

- Class III medical device
- Class C software
- ISO 13485 development
- Analog and digital electronic development Sinus amplifier 1.5Mhz 35 W. Embedded software development
- Customer approval phase Future developments: CE marking and clinical trials
- Small volume production

- Class IIb medical device
- Class B software
- ISO 13485 development FDA
- Brushless direct current (DC) motor with torque and speed
- User interface
- Digital and power electronics
- Circuit board and embedded software, HMI and power-assisted design
- Circuit board production: 500 units per year

- Class IIb medical device
- Class C software
- ISO 13485 and 21_CFR_part_820 development
- Stepper-motor controls Safety-sensor controls HMI Communication
- Design of circuit boards based on STM32 and embedded software – 450 modules – 290,000 lines
- Production exceeds 50,000 per year
International standard monitoring
MAATEL aims to grow their activity in compliance with European and international standards.
We are particularly attentive to our adherence with the latest regulatory requirements.
At Maatel, we apply the following standards:
- EN 60601-1 Standard and derivatives: general standards for basic security and essential performance for electronic devices.
- EN 62304 Standard: medical device software, software life cycle processes.
- 21CFR820 FDA QUALITY SYSTEM REGULATION (QSR) Medical devices
Risk control for your medical device
Criticality Matrix
For any medical device, containing risks is obligatory and stringent attention is required.
Risk analysis is conducted with you throughout your project: this first analysis allows for the identification of the criticality matrix. This matrix is fundamental to identifying critical points for design, and establishing the list of tests to conduct, as well as parameters to respect, either during prototype design, product industrialization or production.
These requirements are applied throughout the complete quality control system at each phase of the product life cycle.
Thanks to our experience in design and mass production of electronic medical devices, MAATEL is the best choice for analyzing the technical risk of your product with the application of stringent standards in monitoring.
Maatel’s expertise in risk analysis and management applies the requirements featured in the ISO 14971 standard for the “Application of risk management to medical devices”.
Version quality control
Applying standards to many medical devices requires your product and prototypes to be versioned.
This involves a range of practices, processes and tools to guarantee traceability of any product evolution, and a record of the complete history and all versions.
Maatel uses two ‘versioning’ tools:
- GFORGE: collaboration management software for tracking product modifications and non-conformities, and associating them with a version.
- Subversion (abbreviation: svn): version management software
Apart from compliance with medical standards, versioning tracks improvements for after-sales service of previous versions of your product, and to plan possible technical improvements.

